







































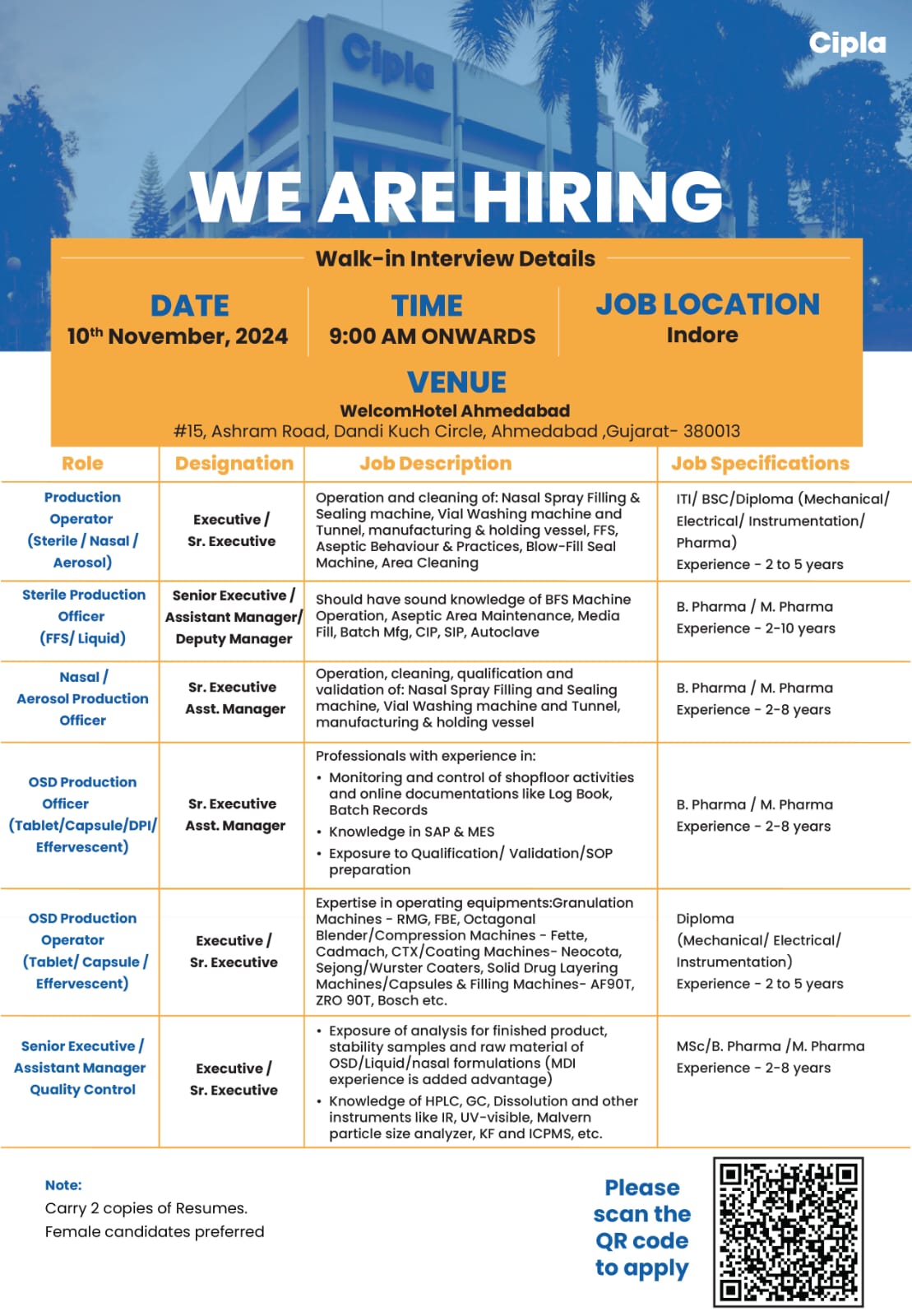

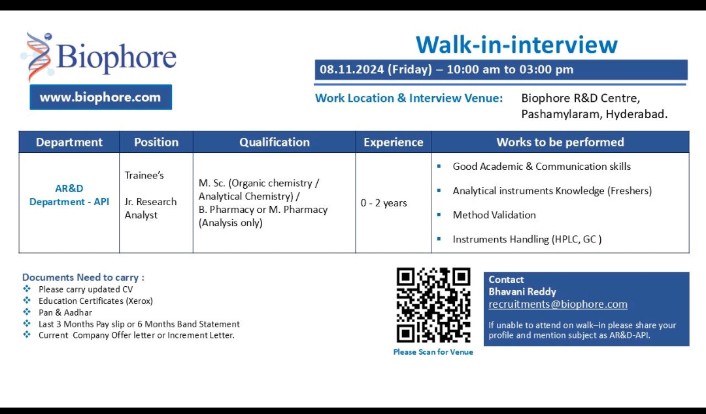

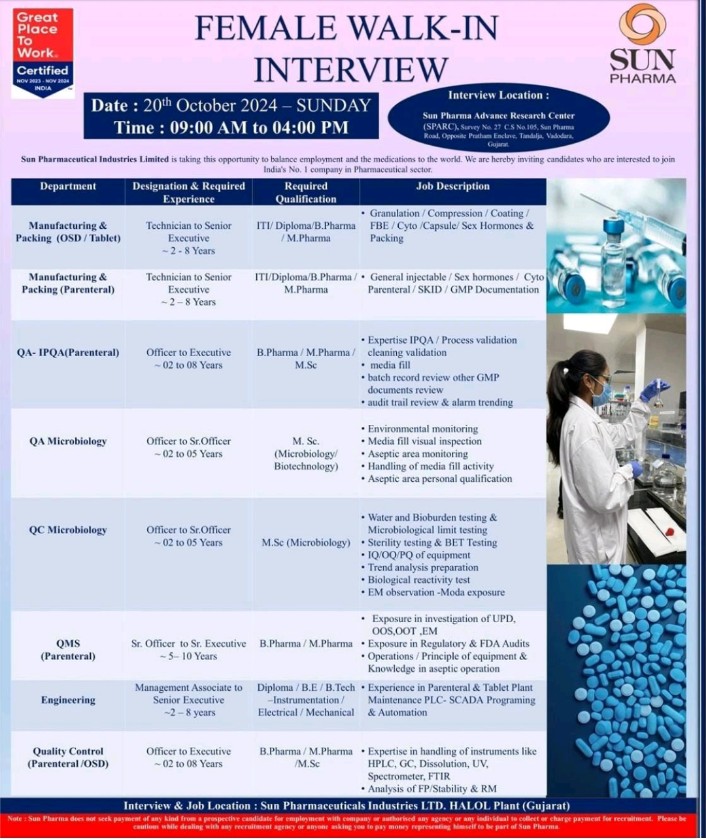





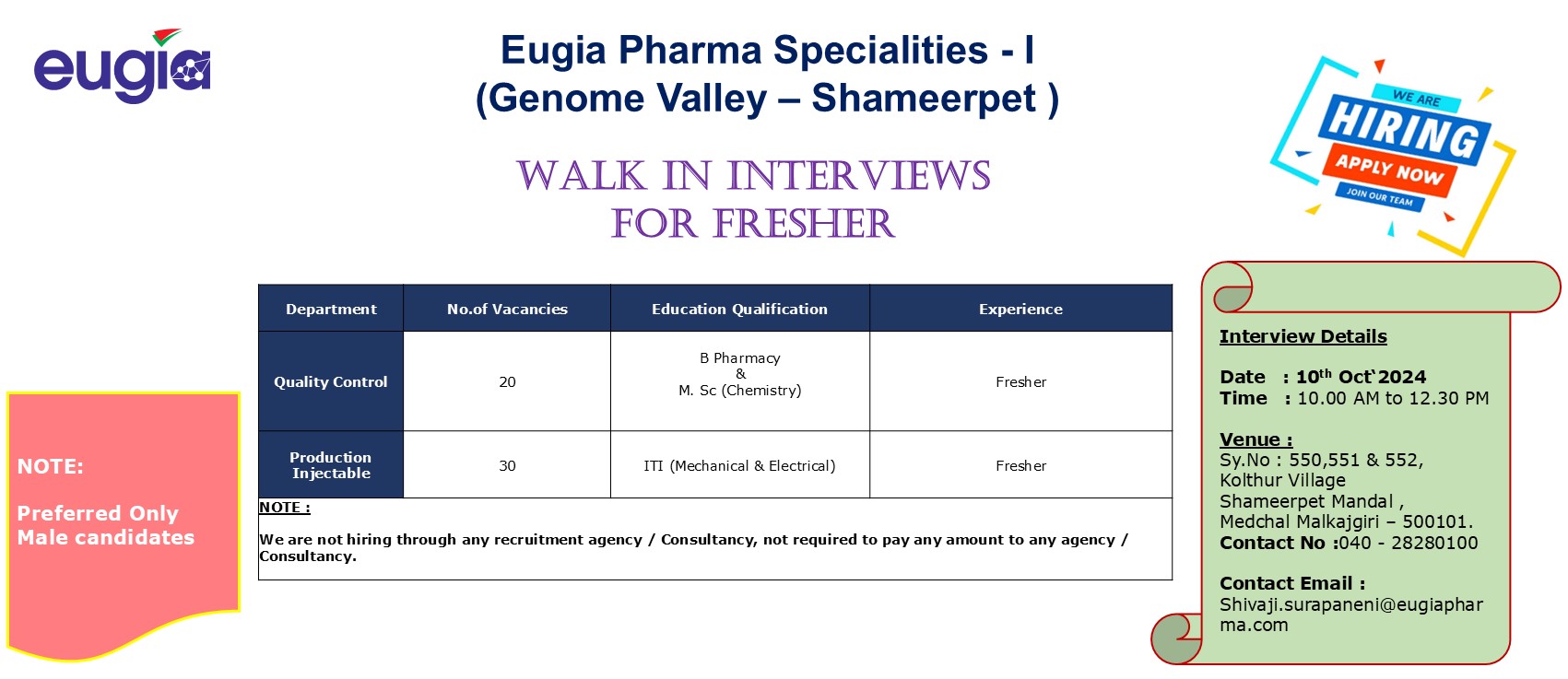

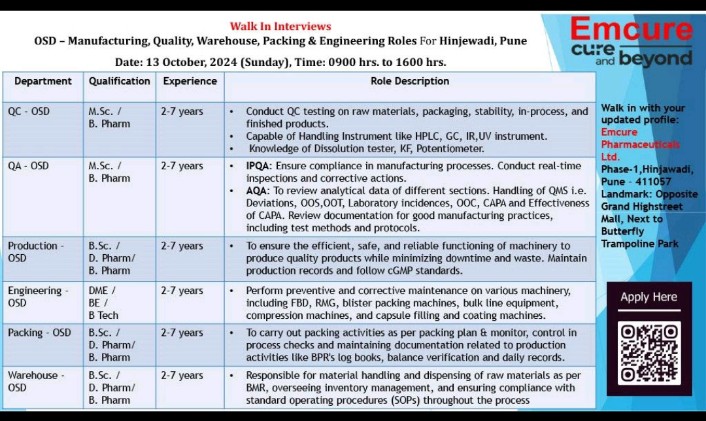


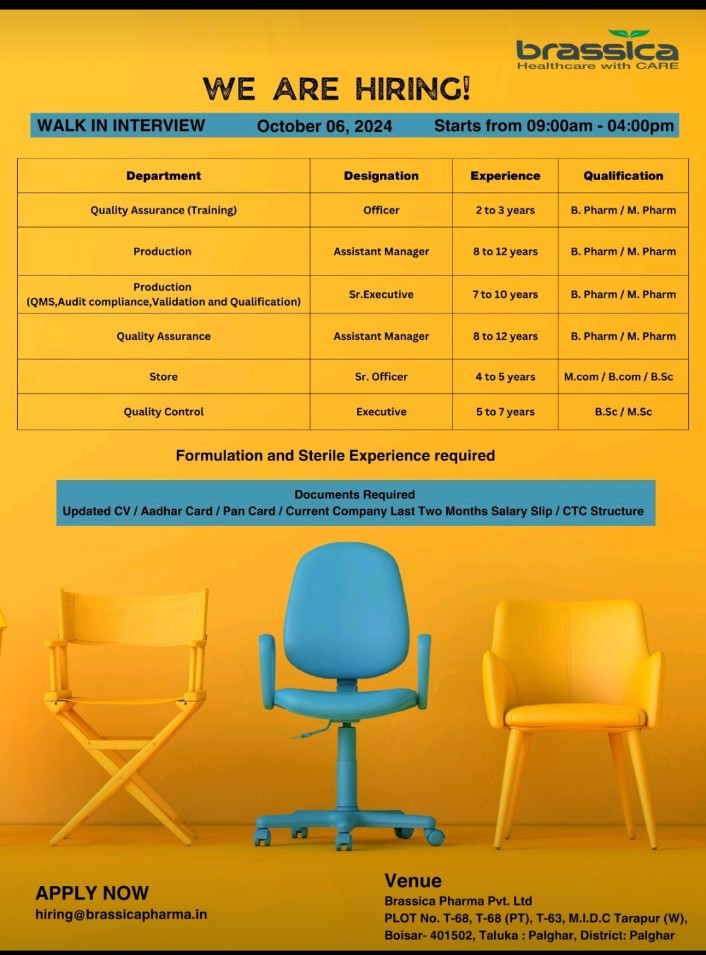
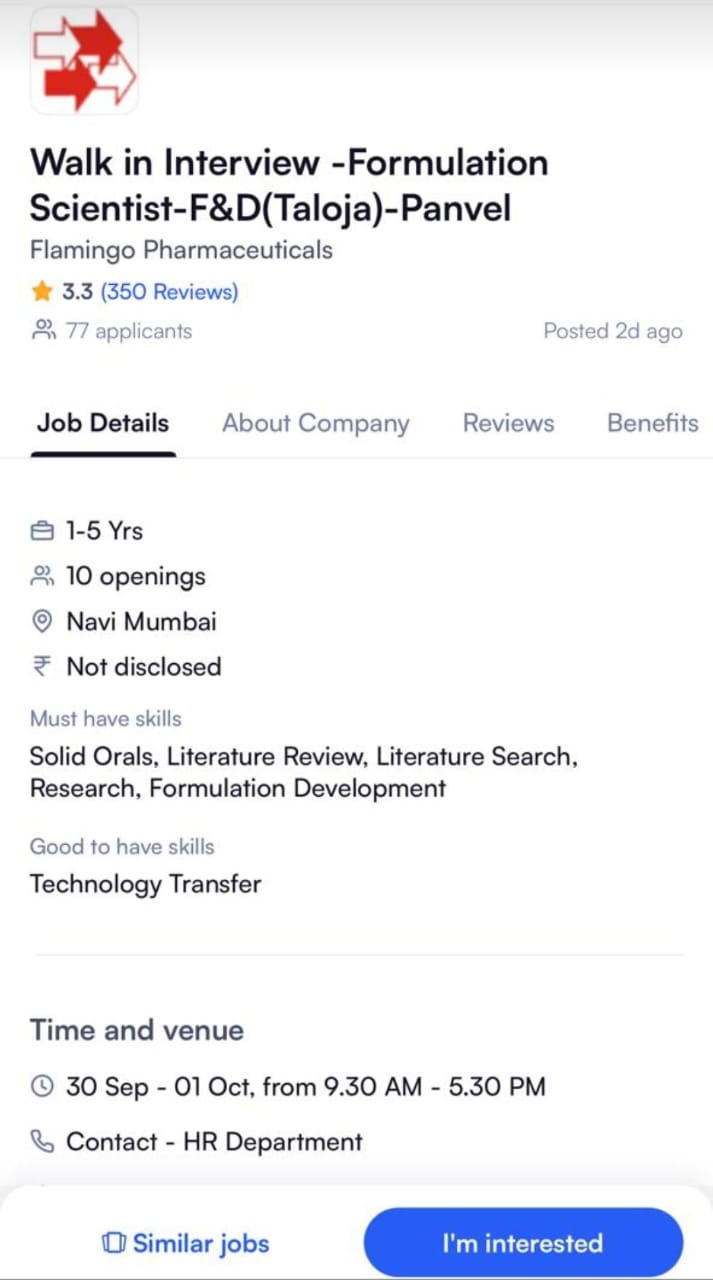


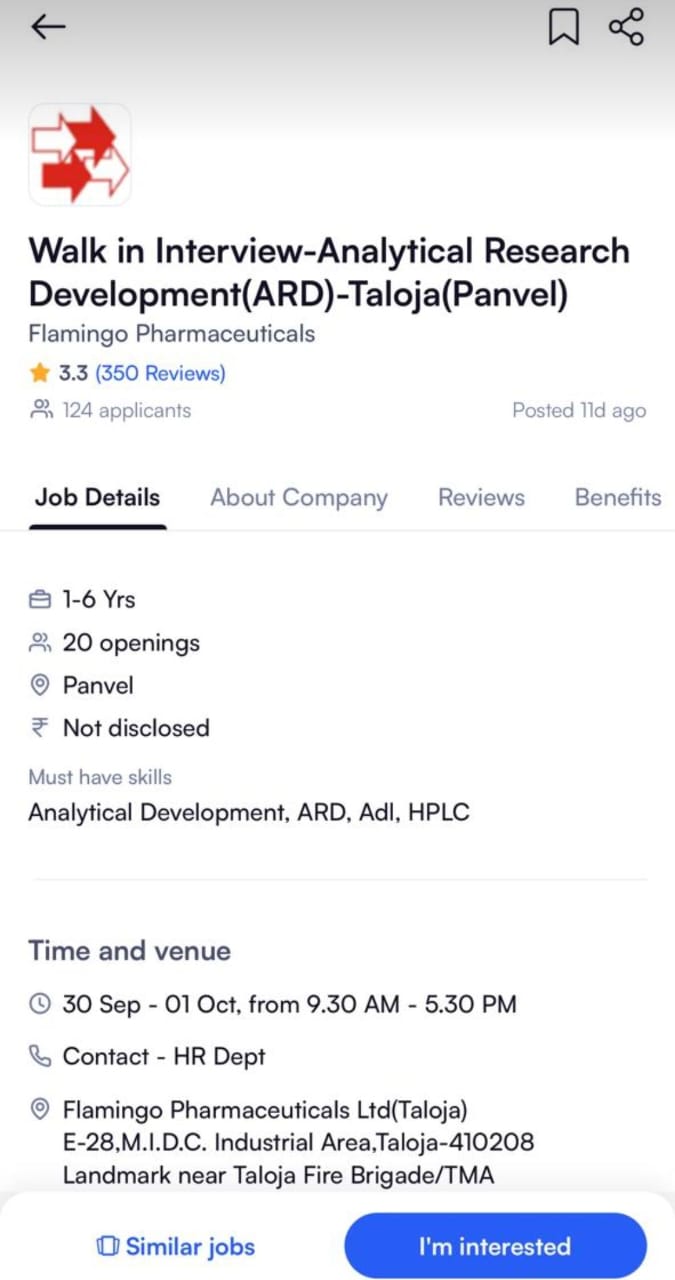



We are hiring! We are looking for talented individuals to join our Regulatory Affairs team. If you have the Passion to Innovate and create an impact on lives, then apply now. Details as below:
● Open Positions:
▹ Sr. Executive – Domestic Regulatory Affairs
▹ Sr. Executive/ Assistant Manager/ Deputy Manager – Global Regulatory Affairs
▹ Sr. Executive/ Assistant Manager/ Manager – Developmental Quality Assurance (DQA)
◉ Qualifications and Experience:
M.Tech / M.Sc in Biology, Biotechnology, Biochemistry, Pharmacy or related field with 6 - 15 years of experience
◉ Job Location: Chakan, Pune
◉ Last date to apply: March 30, 2025
◉ Interested candidates with relevant experience can apply using the QR code or click the link here:
https://shorturl.at/nMc2j
Veeda Lifesciences is Hiring !!
Job Title: Pharmacovigilance Associate
Experience: 0-1 year
Qualification: B.Pharm, M.Pharm, or relevant field
Employment Type: Hybrid
Job Responsibilities:
1-Aggregate Reports:
* Collect safety data and prepare Risk Management Plans (RMP), PBRERs/PSURs, PADERs, and addendums to clinical overview summaries.
* Collaborate with cross-functional teams to resolve issues during safety data review and report preparation.
* Ensure timely submission of reports to meet regulatory compliance.
2-Literature Search & Review:
* Set up search strategies and monitor literature databases for relevant safety information.
* Perform initial review of literature articles, segregating them into ICSRs, AOIs, and other relevant categories.
3-Triaging & Case Processing, Quality Check:
* Receive, track, and triage Individual Case Safety Reports (ICSRs).
* Conduct duplicate searches and ensure case processing aligns with SOPs.
* Review case quality against source documents.
4-Medical Inquiry Management:
* Handle Medical Information Contact Center (MICC) activities.
* Respond to medical, pharmaceutical, and technical inquiries, including adverse events (AEs).
* Maintain records and ensure timely responses to inquiries.
* Report adverse events to the case processing team.
Required Skills:
Knowledge of pharmacovigilance principles and regulatory guidelines.
Familiarity with safety databases and literature search tools.
Strong analytical and problem-solving skills.
Excellent communication and documentation skills.
Interested Candidates can share their resumes at Vaibhav.D3662@veedalifesciences.com or on 6359600877.
Calling All Freshers! Kickstart Your Career with Entry-Level CDM Position at IQVIA!
Are you a detail-oriented individual with a passion for data and healthcare? Join IQVIA as a Trainee CDC and be part of a team that ensures high-quality data management to meet client and project needs.
Job Overview: As a Trainee CDC, you will perform and oversee data management activities, ensuring data is processed and tracked in alignment with regulatory and quality standards.
Essential Functions:
● Conduct basic data review and write data clarifications
● Resolve data clarifications and conduct quality control procedures
● Meet personal project objectives and escalate issues as needed
● Understand and comply with core operating procedures and Data Management Plan
● Develop and maintain good communications and working relationships with the CDM team
Qualifications:
● Excellent organizational, communication, and computer skills
● Strong attention to detail and ability to act independently
● Ability to establish and maintain effective working relationships with coworkers, managers, and clients
If you meet the criteria and are interested, send your CV to richu.joyedakulathur@iqvia.com
Fresher Apprentice recruitment at Zydus Healthcare Ltd. (Ahmedabad R&D)
Department – Analytical Development - Master degree holder in science / Pharmacy.
Department - Project Management – Fresher M Pharma (Pharmaceutics)
Kindly share CV on – Bhakti.Vaghela@zyduslife.com
Date of Posting – 09/01/2025
(Forwarded as received, contact directly)
Career Opportunity with *Sitec Labs Limited*
*Department* - Analytical Redearch Department
*Position Name* - Apprentice in Analytical Dept for 1 year apprenticeship period
*Qualification* - Msc Org/ Analytical
*Number of Position* - 03
*Required Experience* - Fresher
*Job Description* –
Analysis of routine raw material, finished product and excipients samples, Calibration of instrument and equipment.
Should have good theoretical understanding about different analytical techniques/Principles.
Ready to work in shifts
*Job Location* - Mahape, Navi Mumbai.
*Stipend* - As per Apprentice trade and qualification.
Candidates should not have a history of apprenticeship period completion in the past.
After 1 year depends on performance confirmation can be done
*Contact Details*
Intrested candidates can share their updated resume on below email ID or you may share this opportunity with your friends who are looking for better career.
sneha.chavan@siteclabs.com
Join our Global Regulatory Intelligence Team! hashtag#hiring hashtag#fresher
Job Title - Regulatory Intelligence Associate
Experience - 0-1 year
Location- Hyderabad (WFH)
Education- Graduate/ Postgraduate in any life-science related disciplines (B.Pharm/ M.Pharm/ Food tech/ B.Tech/ M.Tech)
Expected Skills -
1. Regulatory Affairs, understanding of global health authorities
2. Communication: Verbal and written communication, presentation skills.
3. Team Collaboration: Teamwork, conflict resolution, adaptability.
Role-
As a RI Associate, you would contribute towards various departmental processes such as collection of regulatory intelligence data, writing of summaries/ synopsis, quality check of data and uploading the data on product platform. These processes are running across the product categories of pharmaceuticals, medical devices and consumer products. Also, the scope of work covers the regulatory intelligence data across all the geographical regions. Depending upon your interest and work exigencies, you would be given an opportunity to specialize in one or more of these functions over the long term.
We invite interested candidates to share their CVs with us at rupali.chakraborty@freyrsolutions.com
About Apotex Inc.
Apotex Inc. is a Canadian-based global health company that produces high-quality, affordable medicines for patients around the world. Apotex employs almost 7,200 people worldwide in manufacturing, R&D, and commercial operations. Apotex medicines are accessible to patients in more than 75 countries globally. Through vertical integration, the Apotex group is focused on the development and sale of generic, biosimilar and specialty products.
For more information visit: www.apotex.com.
Application link:
https://careers.apotex.com/job/Mumbai-Trainee-Quality-Stability-Data-MH-400079/586337817/
Freshers: Walk-In Interview
M.Sc. (Biotechnology/Microbiology/Biochemistry/Life Sciences)
Date: Saturday, 23rd November 2024
Time: 9:30 am to 3 pm
Location: Ipca Laboratories Limited,
470, 471, 481, Sector 3,
Industrial Area, Pithampur,
Dist Dhar, Madhya Pradesh - 454775
Email: piyush.khichi@ipca.com
Google map location:
https://lnkd.in/djEEJ_-e
0 - 2 years, 1.75-3 Lacs P.A.
Job description, Role & responsibilities
REGULATORY RELATED ACTIVITIES :
A. Review and finalization of DCGI required documentation for application in coordination with respective managers.
B. Responsible for BE-NOC, T-license applications & notifications submission to Drugs Controller General of India (DCGI) in coordination with respective managers.
C. To ensure proper query response in stipulated timelines for the submitted applications in coordination with respective managers.
D. To track the regulatory approvals on regular basis and place in common drive of all locations after downloading.
E. Responsible to maintain regulatory tracking sheet of all the submitted applications/approvals/notifications.
NOTE:
Looking for B.Pharma candidates with basic knowledge related to job profile only.
We are not considering M.pharma or any other qualification.
Employment Type: Full Time, Permanent
Education
UG: B.Pharma in Any Specialization
About company
Clinical Research Organization
Address:TP 86, FP 28/1, CLIANTHA CORPORATE , AHMEDABAD, Gujarat, India
dddddddddddddd
Come and join in this enriching journey at Reliance Life Sciences!
Current Openings
Job Description Summary
To assist the formulation development activity.
Job Description
Qualification: M. Pharm Fresher
Commitment to Diversity, Equity, and Inclusion:
At Endo, our diversity unites and empowers us as One Team, and we are committed to cultivating, and valuing, each person’s unique perspective. We actively promote a culture of inclusion that draws strength from our broad spectrums of diversity, including race, ethnicity, religion, gender identity or expression, national origin, color, sexual orientation, disability status, age, and all our other unique characteristics, qualifications, demonstrated skills, achievements, and contributions, backgrounds, experiences, cultures, styles, and talents.
Apply through below link:
https://www.endo.com/careers/open-positions/job?jobid=R001789
We Are Hiring – Join Our Quality Control Team in Nashik!
Are you passionate about ensuring the highest quality standards in pharmaceutical and biologics manufacturing?
We're expanding our Quality Control department and looking for experienced professionals to join our team in Nashik.
Open Positions:
1. Team Lead I (Plasma Products)
o M.Sc. in Microbiology/Biotechnology/Biochemistry or B.Tech/M.Tech in Science
o 21-25 years of QC experience, leading teams of 10-25
2. Team Lead I (Complex Biologics/Biosimilars)
o M.Sc. in Microbiology/Biotechnology/Biochemistry or B.Tech/M.Tech in Science
o 21-30 years of QC experience, leading teams of 20-40
3. Senior Manager (Microbiology)
o M.Sc. in Microbiology
o 15-20 years of QC experience, leading microbiology teams of 5-15
4. Senior Manager (Raw Material and Packing Material)
o M.Sc. in Chemistry
o 15-20 years of QC experience, leading teams of 10-25
5. Analyst Quality Control
o M.Sc. in Microbiology/Biotechnology/Biochemistry/Chemistry
o 2-8 years of QC experience
All roles require expertise in GLP, aseptic practices, and hands-on experience with biologics or plasma products.
Ready to make a difference in global health? Apply now and join our innovative team!
For detailed job descriptions and to apply, visit: https://lnkd.in/dv5Dm-Vi
Job Description Summary
Assistant Manager Regulatory Affairs Labeling will be responsible for Labeling activity/ Submissions for US market.
Job Description
Education and Experience:
M. Pharm/ M. Sc with 6-8 years relevant experience.
Commitment to Diversity, Equity, and Inclusion:
At Endo, our diversity unites and empowers us as One Team, and we are committed to cultivating, and valuing, each person’s unique perspective. We actively promote a culture of inclusion that draws strength from our broad spectrums of diversity, including race, ethnicity, religion, gender identity or expression, national origin, color, sexual orientation, disability status, age, and all our other unique characteristics, qualifications, demonstrated skills, achievements, and contributions, backgrounds, experiences, cultures, styles, and talents
Apply Job through below link:
https://www.endo.com/careers/open-positions/job?jobid=R001269
our career journey."
Reliance Life Sciences Hiring for MSc Microbiology, Biotechnology and Biotechnology.
We Are Hiring - Join Our Plasma Manufacturing and Quality Assurance Team!
About Reliance Life Sciences:
Reliance Life Sciences is a research-driven organization developing business opportunities in bio-therapeutics (plasma proteins, biosimilars and novel proteins), pharmaceuticals (later-generation, oncology generics), clinical research services, regenerative medicine (stem cells therapies) and molecular medicine. Reliance Life Sciences is part of the Promoter Group of Reliance Industries Limited.
We have launched five of the world’s first biosimilars, have the largest number of biosimilars in the market in India, and the highest number of biosimilars under development globally. It has the distinction of being the first integrated manufacturer of plasma proteins in South Asia. Reliance Life Sciences has catalyzed the emergence of regenerative medicine in the country, and has the highest experience with cord blood stem cell transplants in India. RLS is one of the few centres in the world offering a specialized range of tests in molecular medicine and molecular genetics.
Are you passionate about advancing global health through cutting-edge plasma technology? Join our innovative team and make a lasting impact in the field of biopharmaceuticals!
Plasma Manufacturing/Production:
- Roles: Executive/Senior Executive/Deputy Manager
- Qualifications: M.Sc. (Biotechnology/Microbiology/ Biochemistry)
Experience: 2-10 years of experience with expertise in Upstream fractionation, Downstream Protein purification, Industrial Centrifuge, Industrial filtration and fill finish.
- Location: Navi Mumbai / Nashik
Plasma Quality Assurance:
- Roles: Executive/Senior Executive
- Qualifications: M.Sc. (Biotechnology/Microbiology/ Biochemistry)
- Experience: 2-10 years of experience with expertise in QMS, Quality Assurance activities, IPQA, Change control management, OOS and Deviation handling.
- Location: Navi Mumbai / Nashik
Take the next step in your career and help shape the future of medicine. Apply now to become a vital part of our dynamic Plasma Manufacturing and QA team! Click here
Date: Oct 11, 2024
Location:
Navi Mumbai, India, 400706
Company: Teva Pharmaceuticals
Job Id: 58773
Together, we’re on a mission to make good health more affordable and accessible, to help millions around the world enjoy healthier lives. It’s a mission that bonds our people across nearly 60 countries and a rich, diverse variety of nationalities and backgrounds. Working here means working with the world’s leading manufacturer of generic medicines, and the proud producer of many of the products on the World Health Organization’s Essential Medicines List. Today, at least 200 million people around the world take one of our medicines every single day. An amazing number, but we’re always looking for new ways to continue making a difference, and new people to make a difference with.
Manager Regulatory Affairs
Application job link (if link is not opening, copy this link and open in new google window)
https://careers.teva/job/Navi-Mumbai-Regulatory-Affairs-Associate-I-Indi-400706/1221995500/
Clinical Research Associate (CRA)
Mascot Spincontrol India Pvt Ltd
Location: Mumbai, Maharashtra
Job Type: Full-Time
Qualifications:
Experience: 0-1 year
Principal Duties and Responsibilities:
Obtain screening consent from volunteers
Record demographics and fill out habit questionnaires
Maintain screening logs and update the volunteer database
Verify age proof, address proof, and photographs of volunteers
Record details in volunteer enrollment forms and questionnaires
Monitor humidity, temperature, and acclimatization of volunteers
Ensure study flow and obtain informed consent from volunteers
Record and maintain study logs and perform lactic acid stinging tests
Gather proscription/restriction information and subject self-evaluation
Ensure proper data entry, verification, and operation of instruments
Check the quality of CRFs and related documents, ensuring completeness
Archive study-related documents and maintain the registration database
Joining: Immediate
Application:
Send your CV, cover letter, and references (if any) to:
Email: hr@mascotspincontrol.in or nisha@mascotspincontrol.in
Forwarded as received
URGENT JOB OPENING
Calling all Male and Female fresher graduates in MSc Chemistry at Pune
Company: Chem-Tech Laboratories Private Limited
Walk-in Date: Oct 4th and Oct 5th
Position: Trainee Analytical Chemist
Are you passionate about analytical chemistry? Chem-Tech Laboratories is offering exciting opportunities for freshers to kickstart their career journey! Don't miss this chance to showcase your skills and expertise in a dynamic work environment.
Requirements:
MSc in Analytical and Organic Chemistry
- Strong analytical skills
- Attention to detail
- Excellent communication skills
Location: Chem-Tech Laboratories Private Limited S/N 22 Parvati Industrial Area Pune Satara Road Swargate Pune 411009
Time: 10.00 am to 12.00pm
Bring your resume and enthusiasm! Join us and be part of a team dedicated to scientific excellence. See you there!
Stanex Drugs and Chemicals Pvt. Ltd is a prominent player in the pharmaceutical and chemicals industry, dedicated to enhancing health and well-being through innovative solutions. With a strong commitment to quality and compliance, Stanex strives to meet the evolving needs of healthcare professionals and patients alike. The company’s focus on research and development ensures that it remains at the forefront of the industry, providing cutting-edge products that adhere to the highest standards of safety and efficacy.
Responsibilities in Job
As a part of the Regulatory Affairs (RA) Department at Stanex, the key responsibilities include:
Regulatory Documentation: Preparing and reviewing regulatory submissions, including INDs, NDAs, and other compliance documents.
Guideline Compliance: Ensuring that all products comply with national and international regulations and guidelines set by regulatory authorities.
Collaboration: Working closely with cross-functional teams, including research, quality assurance, and production, to ensure seamless communication and compliance throughout the product lifecycle.
Regulatory Strategy: Assisting in the development of regulatory strategies that align with company goals and facilitate product approvals.
Monitoring Changes: Keeping abreast of changes in regulatory requirements and advising the team on necessary adjustments to policies and procedures.
Qualifications
To qualify for a position in the RA Department at Stanex, candidates should possess:
Educational Background: A Master’s degree in Pharmacy (M. Pharm), B.pharm, Msc is essential for this role.
Freshers Welcome: This position is open to fresh graduates
Skills
Candidates should ideally demonstrate the following skills:
Attention to Detail: Strong analytical skills and a meticulous approach to documentation and compliance tasks.
Communication Skills: Excellent verbal and written communication skills to effectively convey complex regulatory information.
Team Player: Ability to collaborate effectively with various teams across the organization.
Problem-Solving Skills: Aptitude for identifying regulatory challenges and proposing viable solutions.
Adaptability: Willingness to learn and adapt to the fast-paced and changing environment of regulatory affairs.
How to Apply
If you are a recent M. Pharm graduate looking to kick-start your career in regulatory affairs, Stanex Drugs and Chemicals Pvt. Ltd invites you to apply! To submit your application, send your resume directly to the HR department via WhatsApp at +91 91542 62431
Emmes Group is dedicated to advancing clinical research and improving patient outcomes through innovation. With over 47 years of experience, Emmes is a leader in clinical research, serving both public-private partnerships and commercial biopharma. They specialize in cell and gene therapy, vaccines, infectious diseases, ophthalmology, rare diseases, and neuroscience. As an Associate TMF (Trial Master File) Specialist, you will play a key role in maintaining and organizing TMF documents while ensuring compliance with industry guidelines.
Primary Purpose
The Associate TMF Specialist is responsible for uploading, indexing, and maintaining TMF documents within an eTMF (electronic Trial Master File) system, ensuring compliance with SOPs, ICH/GCP guidelines, and TMF best practices.
Key Responsibilities
Qualifications
Skills & Competencies
Apply on : https://careers.emmes.com/globalcareers/jobs/2273?lang=en-us
Belstar Microfinance Ltd.
We are welcoming energetic and enthusiastic candidates for Sales Officer Position
Location:- Akola, Murtijapur, Malkapur, Khamgaon,
Jalgaon Jamod, Sindkhed Raja, Wardha, Hinganghat,
Yavatmal, Ralegaon, Nagpur Savner, Umred.
Education – 10th /12th / Graduation
◆Age upto 30
◆ Experience :- Fresher/ Experience
For below position
Sales Officer Education 10/12 or Graduation salary upto 18K Fresher /Experience
Petrol allowance +PF+ Attractive incentives + Group Insurance+ Health Insurance
Note:- Only Immediate Jonier or One Month Notice period Interested Candidates send your Resume Yash Mehare (HR) :- 8805691341
Job Title: Production Trainee
Department: Production
Location: MIDC Kalmeshwar, Nagpur, Maharashtra
Eligibility:
Education: B.Pharm (Bachelor of Pharmacy)
Experience: Freshers welcome to apply!
Walk-In Interview Details:
Date: 29/09/2024
Time: 10 am – 5 pm
Venue: ZIM Laboratories Ltd, MIDC Kalmeshwar, Maharashtra
This is a great opportunity to gain hands-on experience in pharmaceutical manufacturing and launch your career in the industry.
For more details, contact us at careers@zimlab.in